Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Kawaguchi, Munemichi; Miyahara, Shinya; Uno, Masayoshi*
Journal of Nuclear Science and Technology, 56(6), p.513 - 520, 2019/06
Times Cited Count:2 Percentile:21.22(Nuclear Science & Technology)This study revealed melting points and thermal conductivities of four samples generated by sodium-concrete reaction (SCR). We prepared the samples using two methods such as firing mixtures of sodium and grinded concrete powder, and sampling depositions after the SCR experiments. In the former, the mixing ratios were determined from the past experiment. The latter simulated the more realistic conditions such as the temperature history and the distribution of Na and concrete. The thermogravimetry-differential thermal analyzer (TG-DTA) measurement showed the melting points were 865-942 C, but those of the samples containing metallic Na couldn't be clarified. In the two more realistic samples, the compression moldings in a furnace were observed. The observation revealed the softening temperature was 800-840
C, but those of the samples containing metallic Na couldn't be clarified. In the two more realistic samples, the compression moldings in a furnace were observed. The observation revealed the softening temperature was 800-840 C and the melting point was 840-850
C and the melting point was 840-850 C, which was 10-20
C, which was 10-20 C lower than the TG-DTA results. The thermodynamics calculation of FactSage 7.2 revealed the temperature of the onset of melting was caused by melting of the some components such as Na
C lower than the TG-DTA results. The thermodynamics calculation of FactSage 7.2 revealed the temperature of the onset of melting was caused by melting of the some components such as Na SiO
SiO and/or Na
and/or Na SiO
SiO . Moreover, the thermal conductivity was
. Moreover, the thermal conductivity was  =1-3W/m-K, which was comparable to xNa
=1-3W/m-K, which was comparable to xNa O-1-xSiO
O-1-xSiO (x=0.5, 0.33, 0.25), and those at 700
(x=0.5, 0.33, 0.25), and those at 700 C were explained by the equation of
C were explained by the equation of 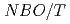 .
.
Ohno, Shuji; Matsuki, Takuo*
JNC TN9400 2000-106, 132 Pages, 2000/12
Sodium fire analyses were performed on 7 kinds of sodium leak tests using a computer code ASSCOPS which has been developed to evaluate thermal consequences of sodium leak accident in an FBR plant. By the comparison between the calculated and the test results of gas pressure, gas temperature, sodium catch pan temperature, wall temperature, and of oxygen concentration, it was confirmed that the ASSCOPS code and the parameters used in the analysis give valid or conservative results on thermal consequences of sodium leak and fire.
Kawaguchi, Munemichi; Miyahara, Shinya; Uno, Masayoshi*
no journal, ,
To evaluate the chemical reaction process of Na-concrete reaction, the model which adopted the thermodynamic database and the latest chemical reaction kinetics in COMSOL Multiphysics has been developed. The chemical reaction processes at each temperature were simulated well by the numerical calculation with this model.
Kawaguchi, Munemichi; Miyahara, Shinya; Uno, Masayoshi*
no journal, ,
no abstracts in English
Aita, Takahiro; Hirano, Hiroshi*; Kimura, Yasuhisa; Shibanuma, Tomohiro; Yoshida, Masato; Nagai, Yuya; Asakawa, Jun; Shuji, Yoshiyuki
no journal, ,
The newly developed Plastic enclosure tents have reliable airtightness and can be set up in a short time with the small number of persons. Also, in order to prevent the spread of contamination, the exhaust device secures the internal airflow line, and the radiation management device measures the concentration of radioactive materials in the air are in real time. Furthermore, by setting up a multiple of evacuation routes, the decontamination time is shortened even when there are many contaminated persons. Therefore, it is possible to quickly evacuate the contaminated person by having both radiation safety and setting up that can quickly respond to a large-scale body contamination accident.
Kato, Saori; Kasugai, Yoshimi; Saito, Fumihiro; Ito, Takashi; Tanaka, Takeshi*; Sugawara, Masakatsu*; Numajiri, Masaharu*; Bessho, Kotaro*; Nakane, Yoshihiro; Miyamoto, Yukihiro
no journal, ,
Three kinds of video materials on the radioactive material leak accident which happened in J-PARC on May 23, 2013 have been produced. These have been screened on the "J-PARC Safety Day" to encourage the J-PARC staff members to keep the memory, to pass on the lessons learned to the next generation, and to use them for future safety.